About COVID-19
What is COVID-19?
COVID-19 is caused by the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a new virus in humans causing respiratory illness which can be spread from person-to-person.
COVID-19 is primarily transmitted from person-to-person through respiratory droplets. These droplets are released when someone with COVID-19 sneezes, coughs, or talks. Infectious droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs. A physical distance of at least 1 meter (3 ft) between persons is recommended by the World Health Organization (WHO) to avoid infection,1 whereas CDC recommends maintaining a physical distance of at least 1.8 meters (6ft) between persons. Respiratory droplets can land on hands, objects or surfaces around the person when they cough or talk, and people can then become infected with COVID-19 from touching hands, objects or surfaces with droplets and then touching their eyes, nose, or mouth. Recent data suggest that there can be transmission of COVID-19 through droplets of those with mild symptoms or those who do not feel ill.
Symptoms of COVID-19
The mostly commonly reported symptoms of COVID-19 include:
- Fever or chills
- Cough
- Shortness of breath or difficulty breathing
- Fatigue
- Headache
- Nasal congestion or runny nose
- Muscle or body aches
- Sore throat
- New loss of smell or taste
- Nausea or vomiting
- Diarrhea
The estimated incubation period is between 2 and 14 days with a median of 5 days. It is important to note that some people become infected and do not develop any symptoms or feel unwell.1
High Risk Populations for COVID-19
The risk of severe disease increases steadily as people age. Additionally, those of all ages with underlying medical conditions (including but not limited to heart disease, diabetes, or lung disease) appear to be at higher risk in developing severe COVID-19 compared to those without these conditions. As more data become available, additional risk factors for severe COVID-19 may be identified.
COVID-19 in Georgia
Daily COVID-19 Rates in Georgia -access current data here
COVID-19 Vaccination Rates in Georgia - GA DPH Vaccine Distribution Dashboard
Preventing COVID-19
Practicing these recommendations will help prevent COVID-19:
- Avoid touching your eyes, nose and mouth
- Avoid contact with anyone who is sick or may feel sick
- Stay at home, and away from others, if you feel sick
- Use a face covering when physical distancing is difficult or when entering closed
spaces
- Physical distancing should be at least 1 meter (3 ft)
- Clean and disinfect frequently touched objects and surfaces often
- Incorporate good hand hygiene into your daily routine:
- Wash your hands with soap and water. The World Health Organization recommends washing hands for 40-60 seconds.
- When hand washing is not available, use a hand sanitizer
- Hand sanitizers should contain at least 60% alcohol2
Vaccines for COVID-19
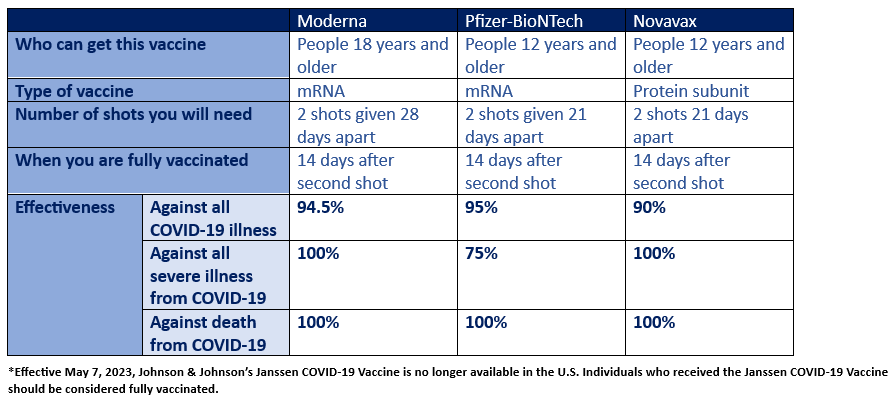
Currently, there are three vaccines that have been authorized in the United States to prevent the spread of COVID-19. Those vaccines include Pfizer-BioNTech, Moderna, and Novavax.
COVID-19 Vaccine Benefits:
- Staying up to date with the recommended vaccines and boosters will ensure that you are best protected from COVID-19
- Updated COVID-19 boosters contribute to restoring protection against newer variants including the most recent Omicron subvariants, BA.4 and BA.5.
The below information explains the different types of vaccination status an individual may have:
Fully Vaccinated
- Individuals are considered fully vaccinated on COVID-19 vaccines if they have completed two doses of COVID-19 vaccine primary series
- Individuals who have a weakened immune system are considered fully vaccinated on COVID-19 vaccines if they have completed their primary series and received the most recent recommended booster dose.
Up to Date
- Individuals are considered up to date on COVID-19 vaccines if they have completed a COVID-19 vaccine primary series and received the most recent booster dose recommended by CDC.
- Vaccine recommendations are based on a person’s age, the first vaccine received, and time since the last dose. People who have a moderately or severely weakened immune system have different recommendations for COVID-19 vaccines.
Vaccine overview:
Individuals are considered fully vaccinated two weeks after receiving their second dose of the Moderna, Pfizer-BioNTech and Novavax vaccines; and two weeks after receiving a dose of the Johnson & Johnson/Janssen vaccine.
Additional vaccines from various manufacturers are under review with the U.S. Food and Drug Administration for use in the United States.
For more information about COVID-19 vaccines click here.
How can I get vaccinated?
COVID-19 vaccines are 100% free. Find a vaccination site near you.
![]() Find a COVID-19 vaccine near you or www.coreresponse.org/covid-19/atlanta-ga
Find a COVID-19 vaccine near you or www.coreresponse.org/covid-19/atlanta-ga
![]() Text your Zip Code to 438829
Text your Zip Code to 438829
![]() Call 1-800-232-0233 (TTY 888-720-7489) or, call the Georgia Department of Public Health
Vaccine Scheduling Line 1-888-457-0186
Call 1-800-232-0233 (TTY 888-720-7489) or, call the Georgia Department of Public Health
Vaccine Scheduling Line 1-888-457-0186
Treatment for COVID-19
At this time, care for patients with COVID-19 is primarily supportive. Care is given to patients to assist in relieving symptoms and managing respiratory and other organ failure. There are currently no specific antiviral treatments licensed for COVID-19, however many treatments are currently under investigation.
- Monoclonal Antibodies - Monoclonal antibodies are laboratory-made versions of proteins
naturally produced by the immune system in response to invading viruses or other pathogens.
Monoclonal antibody therapeutic treatments are available and shipped nationwide. For
more information visit: https://protect-public.hhs.gov/pages/therapeutics-distribution#distribution-locations
- Oral Tablets – Oral tablets have been approved under an emergency use authorization
(EUA) for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults
and pediatric patients (12 years of age and older weighing at least 40 kilograms or
about 88 pounds) with positive results of direct SARS-CoV-2 testing, and who are at
high risk for progression to severe COVID-19, including hospitalization or death.
These tablets should not be considered a substitute for vaccination in individuals
for whom COVID-19 vaccination and a booster dose are recommended. For more information
visit: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19
Please see a health care provider or visit the Test to Treat Program to obtain a prescription.
COVID-19 Testing
There are three types of tests currently available for COVID-19 detection:
Viral Tests
Nucleic Acid Amplification Test (NAAT)
A type of viral diagnostic test for SARS-CoV-2, the virus that causes COVID-19. NAATs
detect genetic material (nucleic acids). NAATs for SARS-CoV-2 specifically identify
the RNA (ribonucleic acid) sequences that comprise the genetic material of the virus.
NAATs can reliably detect small amounts of SARS-CoV-2 and are unlikely to return a
false-negative result of SARS-CoV-2. NAATs can use many different methods to amplify
nucleic acids and detect the virus, including reverse transcription polymerase chain
reaction (RT-PCR). 4
Antigen Tests
Antigen tests are immunoassays that detect the presence of a specific viral antigen, which implies current viral infection. Antigen tests are currently authorized to be performed on nasopharyngeal or nasal swab specimens placed directly into the assay’s extraction buffer or reagent. The currently authorized antigen tests include point-of-care, laboratory-based, and self-tests, and they are applicable to people of any age.
Antigen tests are relatively inexpensive, and most can be used at the point of care. Most of the currently authorized tests return results in approximately 15–30 minutes. Antigen tests for SARS-CoV-2 are generally less sensitive than real-time reverse transcription polymerase chain reaction (RT-PCR) and other nucleic acid amplification tests (NAATs) for detecting the presence of viral nucleic acid.
To sign up receive FREE antigen COVID-19 at-home tests using this link: https://www.covidtests.gov/
Antibody Tests
This type of test is done to determine if a person has previously been infected with the coronavirus disease. Antibody tests should not be used to diagnose a current infection.
COVID-19 Vaccinations in the United States
COVID Data Tracker, Centers for Disease Control and Prevention: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total
What should I do if I test positive for COVID-19?
Isolate - If you test positive for COVID-19, it is recommended that you stay home for at least 5 days and isolate from others in your home. During the first 5 days you are more likely to be infectious. While isolating it is important to wear a high-quality mask if you must be around others at home and in public. Isolating means to remain home and away from everyone else including those in your household, if possible. This is done by remaining in one room in your residence for the amount of time you have been directed to isolate. If you must share a bathroom, all surfaces of the bathroom must be sanitized after use. Click here for more information.
What should I do if I have been exposed to someone who has COVID-19?
If you have been exposed to someone who tested positive for COVID-19 or have been contacted by a health official about being exposed, below are preventive measures that you should take regardless of your vaccination status:
- If you do not have symptoms related to COVID-19, you should begin by isolating for 5 days
- If you do have symptoms related to COVID-19, you need to isolate immediately, get tested, and stay home until you know the result. If your test result is positive, follow the isolation recommendations.
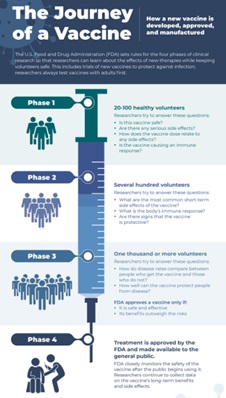
Clinical Trials
 View Larger Image |
Clinical trials allow researchers to test new ways to prevent, detect, or treat disease. Treatments might be new drugs or combinations of drugs, new surgical procedures or devices, or new ways to use existing treatments. Clinical trials can also test other aspects of care, such as ways to improve the quality of life for people with chronic illnesses.6 Clinical Trials were used to test the efficacy of all emergency use authorization COVID-19 vaccines to ensure they were safe for use. COVID-19 Clinical Trials COVID-19 Prevention Trials Network CoVPN 3006- Prevent COVID U -Trial Information l Flyer Children and Youth -Trial Information CoVPN Volunteer Screening Registry -Register Here |
Sources
- Advise on the use of masks in the context of COVID-19external icon. 5 June 2020.
- Guide to local production: WHO-recommended handrub formulationsexternal icon. April 2020.
- COVID-19 Overview and Infection Prevention and Control Priorities in non-US Healthcare Settings. February 2021.
- Nucleic Acid Amplification Tests (NAATs). 14 June 2021. https://www.cdc.gov/coronavirus/2019-ncov/lab/naats.html
- Interim Guidance for Antigen Testing for SARS-CoV-2. 13 May 2021. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html
- WHY DO RESEARCHERS DO DIFFERENT KINDS OF CLINICAL STUDIES, National Institutes of Health, 2021. https://www.nih.gov/sites/default/files/health-info/clinical-trials/infographic-why-researchers-different-kinds-clinical-studies.pdf
- “CDC COVID Data Tracker.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, covid.cdc.gov/covid-data-tracker/#vaccinations
- Total Cases And Weekly Trends, By State And Territory, NPR, 1 Aug. 2021, www.npr.org/sections/health-shots/2020/09/01/816707182/map-tracking-the-spread-of-the-coronavirus-in-the-u-s
- COVID-19 Vaccinations in the United States. COVID Data Tracker. Centers for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total